Healthier Employees,
Stronger Business.
With over 20 years of experience, Discovery Health Services is your trusted partner in responding to and preventing public health crises.
Rapid Response and Prevention for Health Crisis.
At Discovery Health Services, we excel not only in crisis management but in fortifying your workforce’s well-being. We believe that thriving requires more than offering testing and services; it’s about nurturing your organization for a successful year, even when adversity strikes.
Our dedication is to redefine success, measuring it not by the crises encountered but by the resilience displayed amidst them. We’re committed to delivering more than just health crisis solutions; we aim to create a workplace where health is a constant, preventing crises from infiltrating the workplace.
Soften the impact of public health crises with our trusted programs.
Unrivaled Public Health
On-Site Health
Services
Flu Vaccination
Programs
Navigating Mental
Health Crisis
At the heart of a public health crisis, emotional and mental health challenges often impact productivity in the workplace. Beyond handling infectious diseases, we offer a wide range of essential wellness services, promoting peace and wellbeing among your team, empowering them to navigate through crises with resilience and maintain a harmonious work environment.
Safeguard Health with
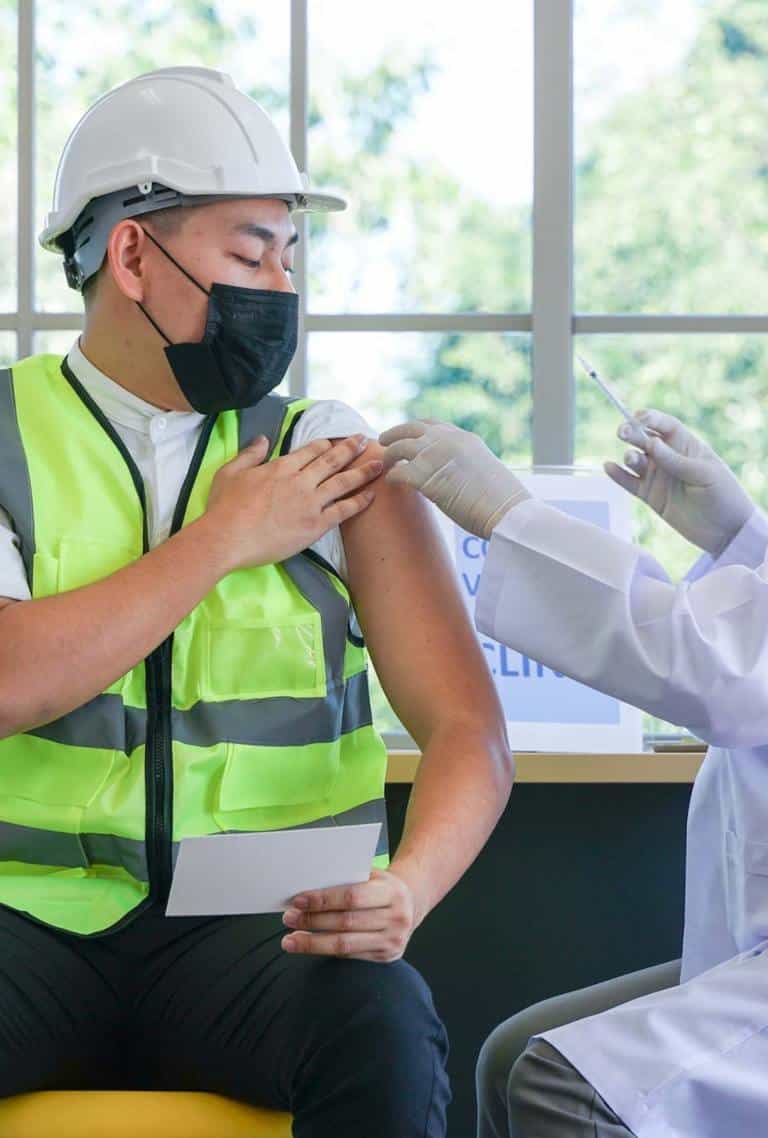
Amidst a public health crisis, every moment counts. Our expertise in logistics allows us to streamline the vaccination process, ensuring a smooth and well–organized distribution of vaccines to your workforce.
Don’t make the mistake of telling your employees to find testing on their own in order to come to work. Not only does this add a level of stress and chaos to the idea of testing–but you won’t be able to control the quality of the test or how it’s taken to ensure accurate results.
Safeguard your workforce and avoid disruption.
Offer easy on – site testing - Reduce employee stress
- Minimize exposure and spread of illness
- Ensure accurate results
- Avoid major workforce disruption
Why Partner with Us for Public Health Crisis Management?
We foster a safe and thriving work environment during the most challenging times.
Understanding the intricacies of workplace dynamics means recognizing that it’s not just about what we do but the environment we make. Our tailored, technology-driven corporate wellness programs go beyond generic solutions to implement a plan that actively engages and keeps employees feeling safe and ready to perform no matter what’s going on outside.
It’s more than just offering services – we work with you to create the right messaging that relieves workers’ anxiety and communicates an understanding of their needs. Your workplace should be a safe haven where your employees feel supported, and their well-being is the top priority.
Technology – driven solutions On – site workplace health solut ions - Vetted medical staff
Securing Health Across
All Industries.
Each industry faces unique challenges when it comes to public health crisis management. That’s why our solutions are tailored to fit the needs of any business or organization, regardless of size or sector.
From testing to vaccinations and compassionate care, our team is dedicated to safeguarding your workforce. Regardless of your team’s field, we stand ready to deliver the services they need, ensuring their well–being is our top priority.
Our solutions are efficient and effective, tailored for your exact needs.
- Government Agencies
- Manufacturing Companies
- Schools and Universities
- Office Environments
- Agricultural Industries
With 20+ years of experience, you can be confident in your workforce’s health.
Your Trusted Partner for
Safeguard your
Workplace.
Avoid major disruption and proactively manage public health crises!
PROUDLY SERVING THE BEST
Trusted By Industry Leaders